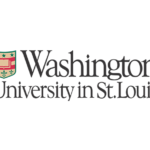Complete Omics Inc. proudly offers a hands-on CLIA/CAP training program designed to empower laboratory professionals, graduate students, and researchers with the skills and knowledge necessary to operate in certified clinical laboratory environments. Anchored by our deep expertise in clinical proteomics and regulatory compliance, this program is led by our Medical Director, Dr. Naseruddin Höti, and our Scientific Team. This 8-week training course immerses participants in real-world laboratory workflows under CAP and CLIA regulations, covering essential procedures such as sample accessioning, mass spectrometry-based clinical assay development, toxicology and pharmacogenomics screening, quality control documentation, and regulatory audit preparation. Each session combines theoretical knowledge with practical execution, ensuring that trainees gain both regulatory awareness and technical proficiency. At Complete Omics, we integrate this training within our operational CAP-accredited and CLIA-certified laboratory—home to cutting-edge diagnostic assay platforms like Complete360® and Valid-NEO®—giving participants access to real patient-derived samples, validated clinical SOPs, and industry-leading instrumentation. This program mirrors the highest standards in laboratory medicine, drawing from our experience in supporting major national and international projects such as CPTAC, the APOLLO Network, and the International Cancer Proteogenome Consortium (ICPC). Trainees completing this program will leave with the capability to contribute to diagnostic assay development, clinical data generation, and laboratory compliance in regulated environments. Participants will also be equipped with templates and tools for traceable documentation, instrument log maintenance, and QA/QC practices required for successful CLIA/CAP certification and audits. All training modules are supplemented with Complete Omics’ proprietary documentation templates, data recording sheets, and compliance tracking systems. Upon course completion, trainees will receive a formal certificate of training along with all supporting materials needed to assist in CLIA/CAP accreditation efforts for their home institutions and for their job huntings. Follow-up consultations are also available free of charge for those pursuing their own certifications or implementing compliant workflows. We welcome you to join us at Complete Omics Inc. to gain the practical skills, regulatory literacy, and confidence to contribute meaningfully to the future of clinical laboratory science. 1, Graduate Students and Postdoctoral Researchers Especially those in biomedical sciences, clinical laboratory sciences, bioinformatics, or molecular diagnostics, who are seeking hands-on training to bridge academic research with real-world clinical diagnostics, and Looking to strengthen their resumes for jobs in regulated clinical labs or biotech companies 2, Laboratory Technicians and Clinical Scientists working in research or hospital settings who want to transition into CLIA/CAP-compliant environments and aiming to improve understanding of QA/QC protocols, documentation standards, and regulatory compliance 3, Hospital and Clinical Lab Professionals Staff from hospital-affiliated laboratories interested in achieving or maintaining CAP/CLIA certification, who are looking to develop new clinical tests or improve existing assay workflows within regulatory frameworks 4, Lab Directors and Managers Wanting to upskill their teams on CLIA/CAP standards and are Interested in preparing their labs for CAP inspections or CLIA audit readiness 5, International Researchers and Professionals from countries without existing CLIA/CAP frameworks who want to adopt U.S. gold standards in clinical lab operations and are interested in bringing CAP/CLIA-compliant workflows and knowledge back to their home institutions or companies 6, Entrepreneurs and Start-Up Founders in Diagnostics who are building clinical testing companies and needing a strong foundation in CLIA/CAP regulatory science and wanting to understand operational compliance before applying for CLIA licenses Not sure if this is right for you? Please contact us to discuss. June 25, 2025 | BALTIMORE – We are thrilled to share that a major milestone in cell therapy has just been published in Cell, showcasing the world’s first-in-human clinical study of… June 5, 2025 | BALTIMORE -Complete Omics Inc. is proud to announce a successful debut at the American Society for Mass Spectrometry (ASMS) 2025 Annual Meeting, held in Baltimore, Maryland. Feb 20, 2025 | BALTIMORE – Complete Omics Inc., a leader in clinical proteomics and multi-omics molecular diagnostics, proudly announces its recent achievement of the California Clinical Laboratory License. This…
Introduction:
People we accept:
Complete360® Enables Deep Biomarker Profiling in Groundbreaking iPSC CAR‑NK Cell Therapy Study Published in Cell
Complete Omics Makes Landmark Debut at ASMS 2025
Milestone! | Complete Omics Inc. Secures California Clinical Laboratory License, Expanding Access to Cutting-Edge Clinical Proteomics Testing
Some of our impacts
2023–Closing latest financing round with QiMing Venture Partners
Complete Omics proudly announces its latest financing round’s success, significantly backed by Qiming Venture Partners, China’s top healthcare VC. This achievement is particularly remarkable given the current economic climate, where securing investment has become increasingly challenging, especially in the healthcare and pharmaceutical sectors. Our team’s dedication and resilience have been key in reaching this pivotal moment for our company. Our collaboration with Qiming, known for investing in industry leaders like Xiaomi (1810.HK), Meituan (3690.HK), and Bilibili (BILI), signals strong confidence in our mission and future. This investment propels us into new realms of innovation in clinical proteomics and molecular diagnostics, underscoring our commitment to transforming healthcare. It positions us among influential firms, promising a future of growth and breakthroughs despite the economic slowdown. We’re excited for what’s ahead and invite our community to join us in this journey towards significant healthcare advancements. Together, we’re poised to redefine the landscape of personalized medicine. (Ref. 1, 2).
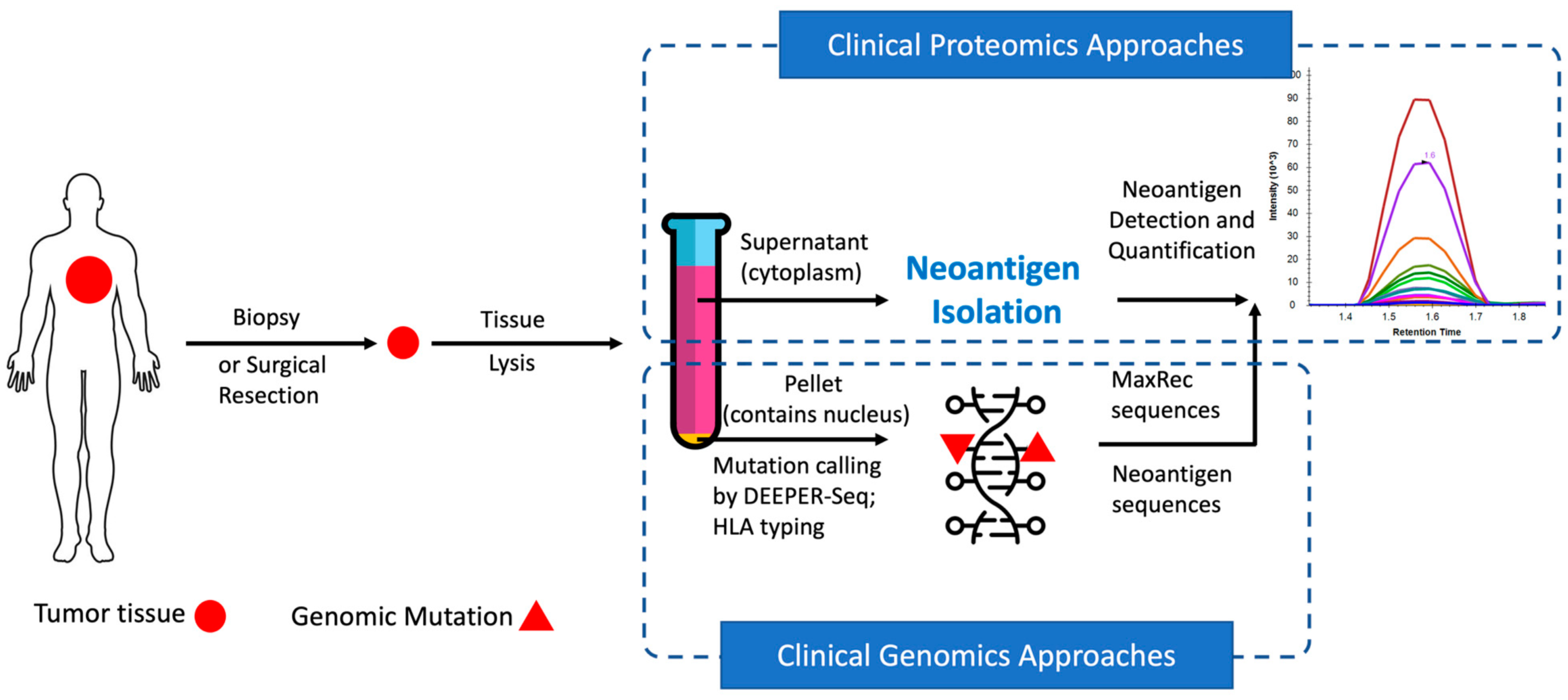
2022–Direct Identification and Quantification of Neoantigens from Minute Amount of Clinical Biopsy Sample — published on Cancers
In recent years, neoantigens are becoming popular cancer therapeutic targets under intensive studies by almost all major oncology pharmaceuticals. However, who are the target patients? Does the patient with a “correct” mutation and a “correct” HLA allele indeed present the “correct” neoantigen? Is this individual’s neoantigen copy number high enough for immunotherapy? AI predictions based on NGS genomic information have been proven incapable of answering these questions. Immunopeptidome through mass spectrometry is dominated by disease-irrelevant peptide sequences. There is no existing way to identify and quantify neoantigens from a minute amount of clinical biopsy sample, such as 50 mg tissue or less. Valid-NEO is developed to fit this demanding clinical need through combining our proprietary multi-omics platforms including NGS-based ultra-rare mutation calling technique, DEEPER-SeqS, and our unique clinical proteomics platform, Complete360®, with additional hardware innovations (Ref. 1).
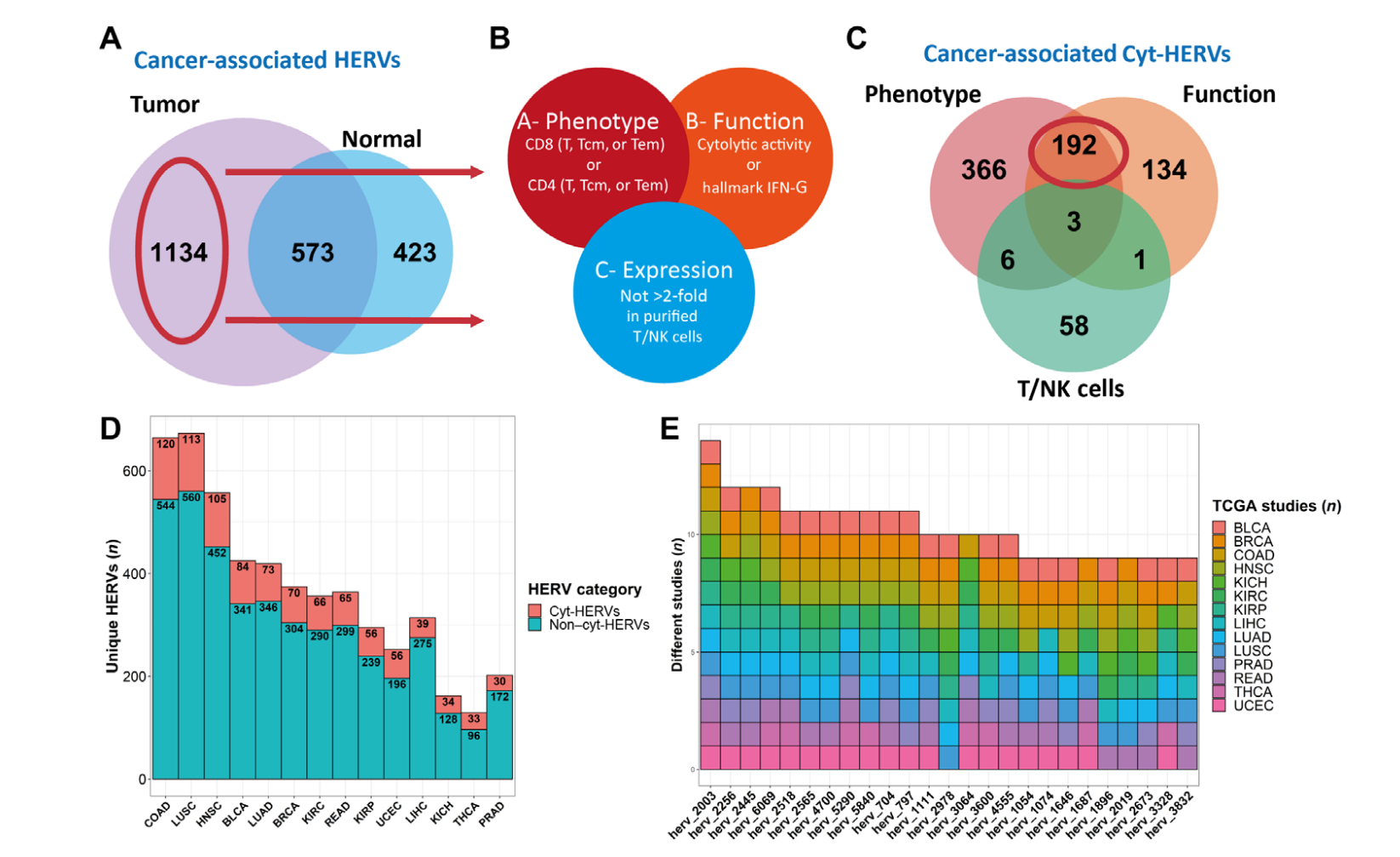
2022–Identify and Quantify Neoantigens derived from Human endogenous retroviruses (HERVs) — published on Science Advances
Human endogenous retroviruses (HERVs) represent 8% of the human genome. Working with, ErVaccine Technologies, our scientists identified neoantigens encoded by HERVs across a broad spectrum of cancers, and this finding may help enable next-generation therapeutic vaccines and cellular immunotherapies targeting these so-called “unconventional” tumor antigens (Ref 1). These antigens are shared by different tumor types. They would prove useful as personalized cancer therapeutic targets for a large number of patients.
Along the side when we are keep on improving and developing our own disease detection and companion diagnostic pipelines, we are excited to be working with a large number of collaborators to implement our multi-omics platforms in their different clinical and basic research projects.
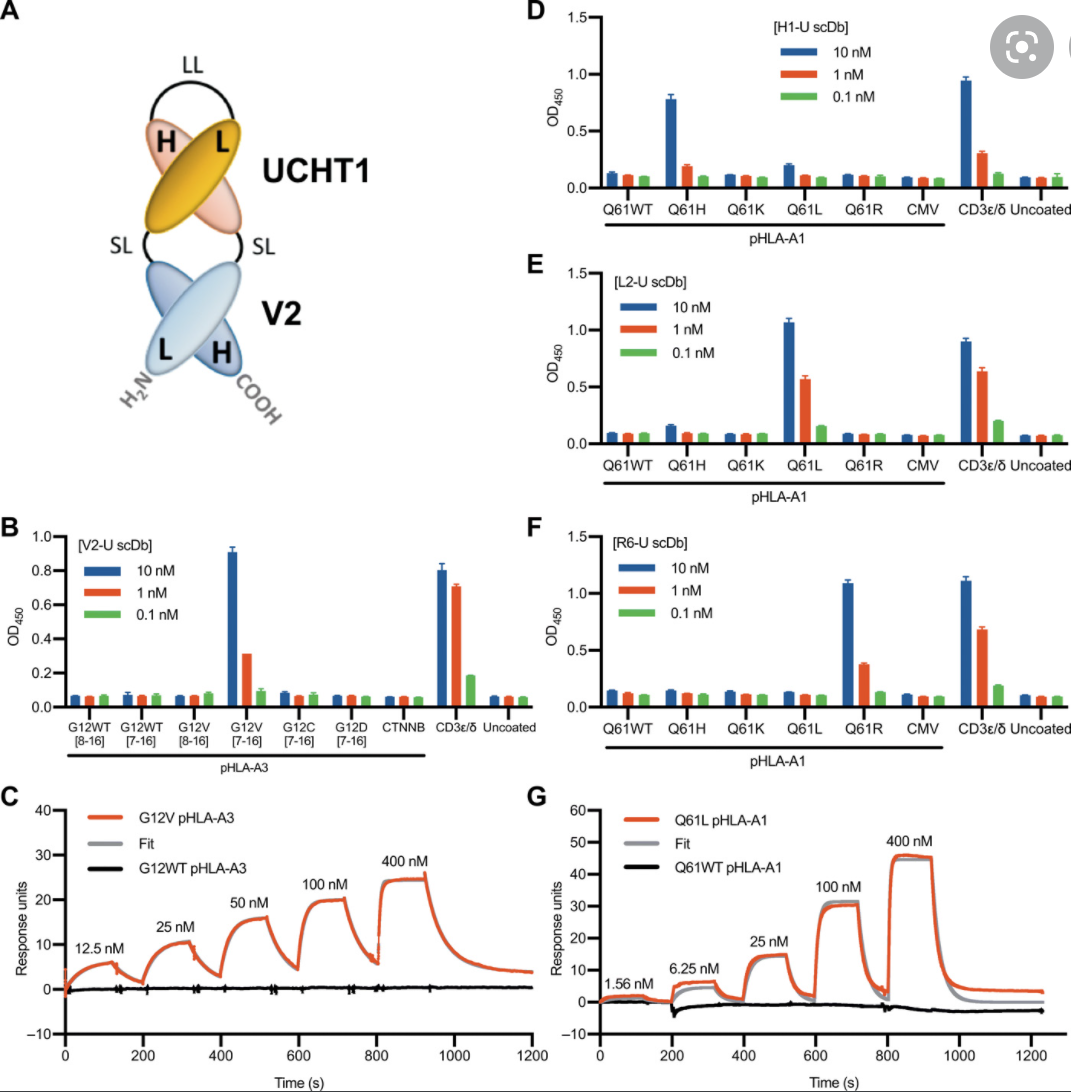
2021–Therapeutic Neoantigens Encoded by Oncogene K-Ras — published on Science Immunology
K-Ras is one of the most highly mutated oncogenes in cancers. The neoantigens encoded by K-Ras can be presented by many different types of cancer cells. Here we utilized our multi-omics neoantigen validation pipeline to detect and quantify K-Ras neoantigens from a variety of cancer samples (Ref 1.). We found that the K-Ras as well as several other oncogenes can be presented on cancer cell surface, but at extremely low copy numbers. We adopted a variety of internal control system to measure their abundance down to <1 copy per cell level. To come up with an actionable strategy, the therapeutic team at JHU developed bispecific antibodies that can target the neoantigens we identified, and deliver dramatic therapeutic effects to mice. These findings are significant in the way that it opens a gate to developing pan-cancer immunotherapeutic agents that can treat a large number of patients sharing cancer hotspot mutations. Identifying such neoantigens is the first step in this campaign and is being accomplished by Complete Omics Inc.
2021–Direct Quantification of Neoantigens Like Never Before — published on Science
Genetic changes in human genome are the driving force for all cancers. Different patients have different sets of mutation profiles even for the patients who all have the same disease. For decades, doctors, cancer researchers, and pharmaceutical companies have been working tirelessly trying to find a way to treat each person’s unique disease in a highly personalized way that will reach the maximum treatment efficacy with the lowest side effects. Complete Omics, working with leaders in cancer therapeutics, has developed pipelines based on our multi-omics techniques through which we clearly observe and quantify personalized therapeutic targets encoded by the most frequently mutated tumor suppressor gene TP53. We validated and quantified the TP53 neoantigens on the surfaces of cancers and provided information to healthcare providers to support their decision on if or not to adopt a highly personalized cancer treatment targeting these neoantigens and when to use it (Ref 1). These findings provided the 1st-hand evidence for cancer therapeutics without the uncertainty that comes with predictions.